



Insects of note, 2024 EditionI average about 1500 photos per year, mostly insects, which I post to iNaturalist for ID. On this page I record the subset that I find interesting, or particularly beautiful. The organization is reverse-chronological, with the oldest material (from Oregon) below the recent, mostly eastern observations. Many of these images are focus-stacked - see details. David Cappaert, Quamash EcoResearch. Find the 2025 edition here. Click any image for a larger magnification. |
Taxonomy projects |
Second tranche of specimens from Rufus Isaac's lab at MSU: Andrena, male and female exemplars. I've used this material to update the Andrena guide.

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
Seed predatory weevils and Sidalcea spThe checkermallows--Sidalcea sp--are prominent in prairies of the Pacific Northwest, and important in restoration ecology. However there is a significant obstacle to seed production: predation by weevils, predominantly Macrorhoptus sidalceae (background here). I am working with Brooklyn Richards of Oregon State University to document the weevil species, and the parasitoids that attack them. Photos here are of the species collected from Sidalcea campestris seed in 2024. Parasitoid IDs are challenging. My shortcut was to ask Jeong Yoo, a Chalcidoidea specialist on iNat. I am sending her additional photos to narrow down the diagnoses. |

The dominant weevil species, Macrorhoptus sidalceae. Traits of Macrorhoptus (page 284).
|

Macrorhoptus - diagnostic details.

|

Anthonomus melancholicus, based on the host association, and consistent with key in The North American Species of the Anthonomus squamosus Species-Group. iNat comments agree. Note metatibial spine, subequal to tarsal claw.
|
Morphotype E - Melotobus sp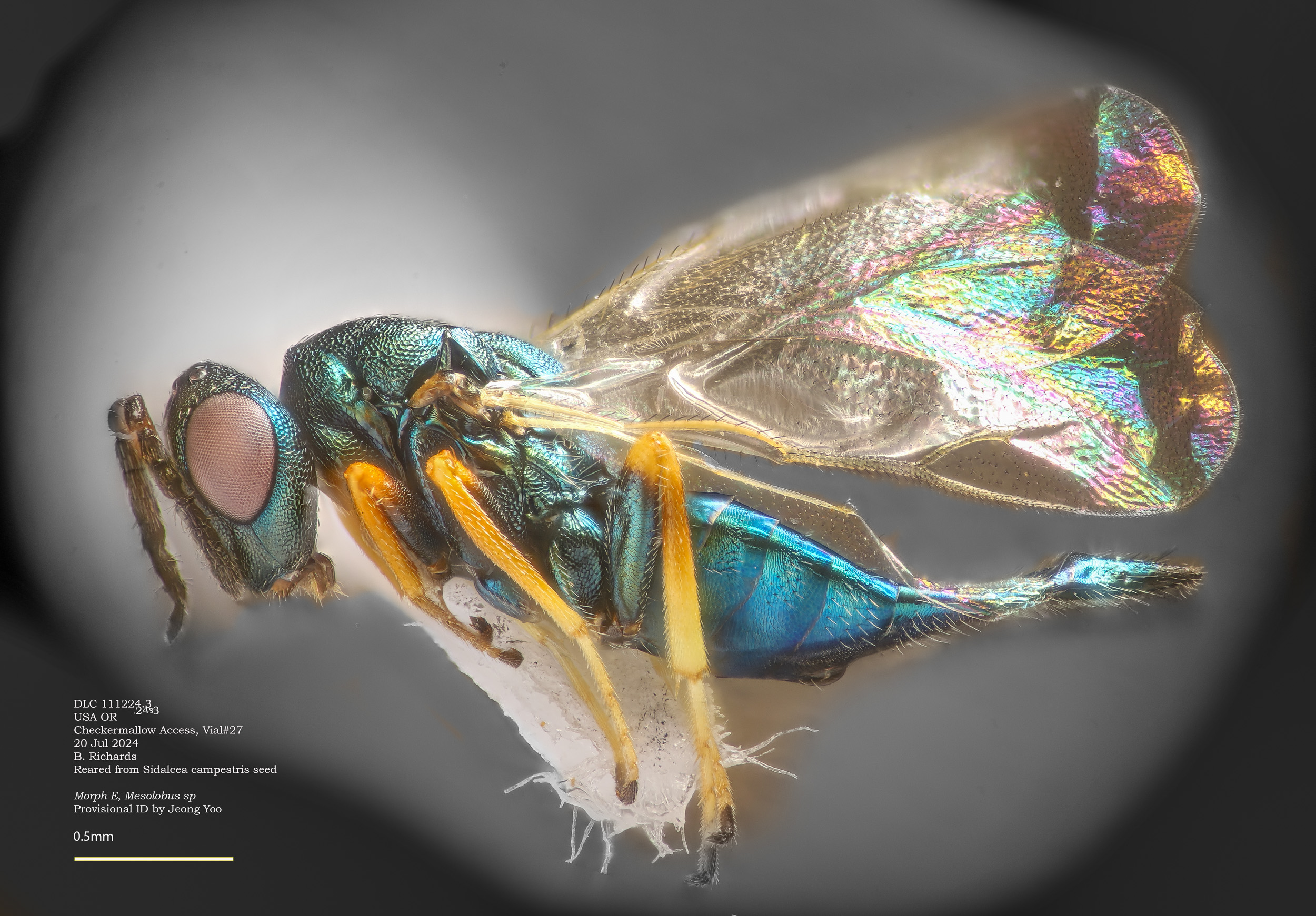
Far the commonest parasitoid observed, "morphotype E" provisionally ID'd by Jeyong Yoo as Melotobus sp.
|

Head of specimen at left. Head of second specimen below.

|

Dorsal view of morphotype E (Melotobus sp); oblique view below.

|
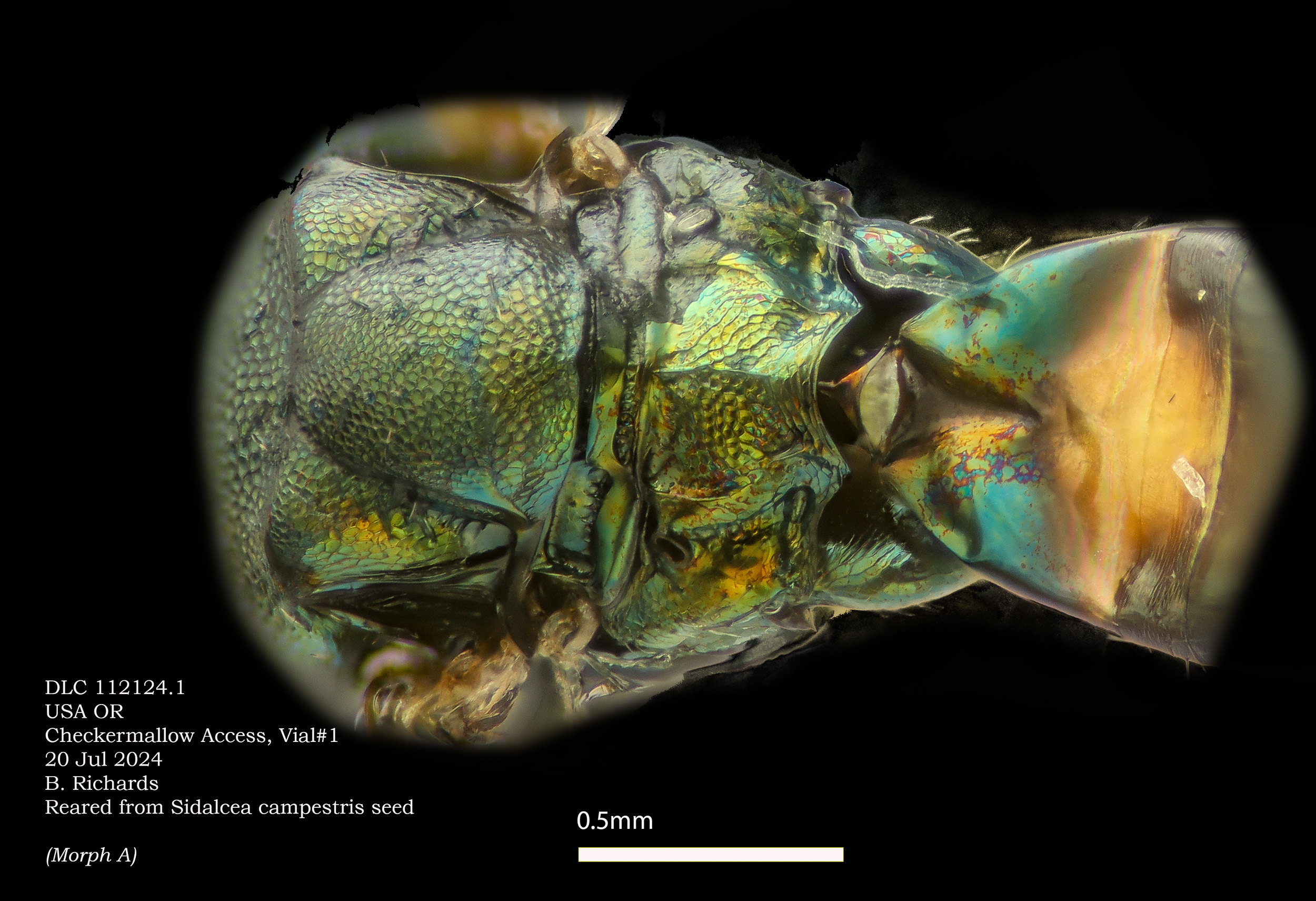
Morph A. Pteromalus sp

Below: different specimen of Morph A.
|
Morph K. Baryscapus sp

Morph J - Telenomus sp
Morph J = Telenomus sp. The genus includes parasitoids of heteroptera, lepidoptera, diptera and neuroptera. See info on genus. "Telenomus is by far the largest genus in the subfamily and includes a considerable number of species that cannot be reliably identified." It will be difficult to verify the host, weevil or by-catch.
|
Morph D - Melittobia spNote from Jeong: I'm skeptical of its origin ... it is a Melittobia sp. (Eulophidae, Tetrastichinae), and they are parasitoids of aculeates or their parasitoids.


|
Brachycyrtus ornatus
Ichneumonidae. Members of the genus are parasitoids of Neuroptera, i.e., this species is by-catch. So, caution: don't assume that every parasitoid has a weevil host.
 |
Morph C - Brasema sp

|
Morph N - Also Brasema sp

 |
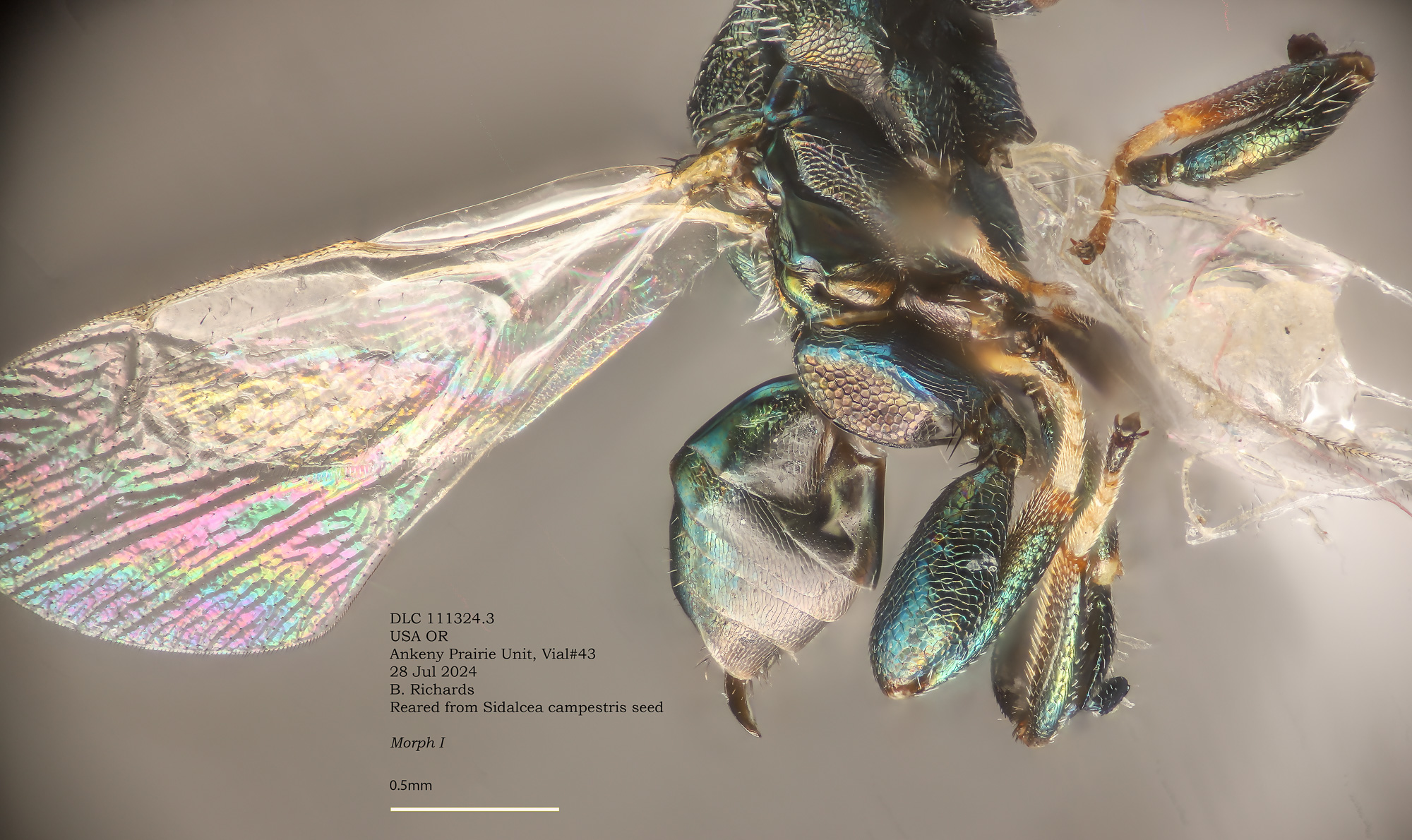
Morph I, L - Torymus sp
Morph I
|
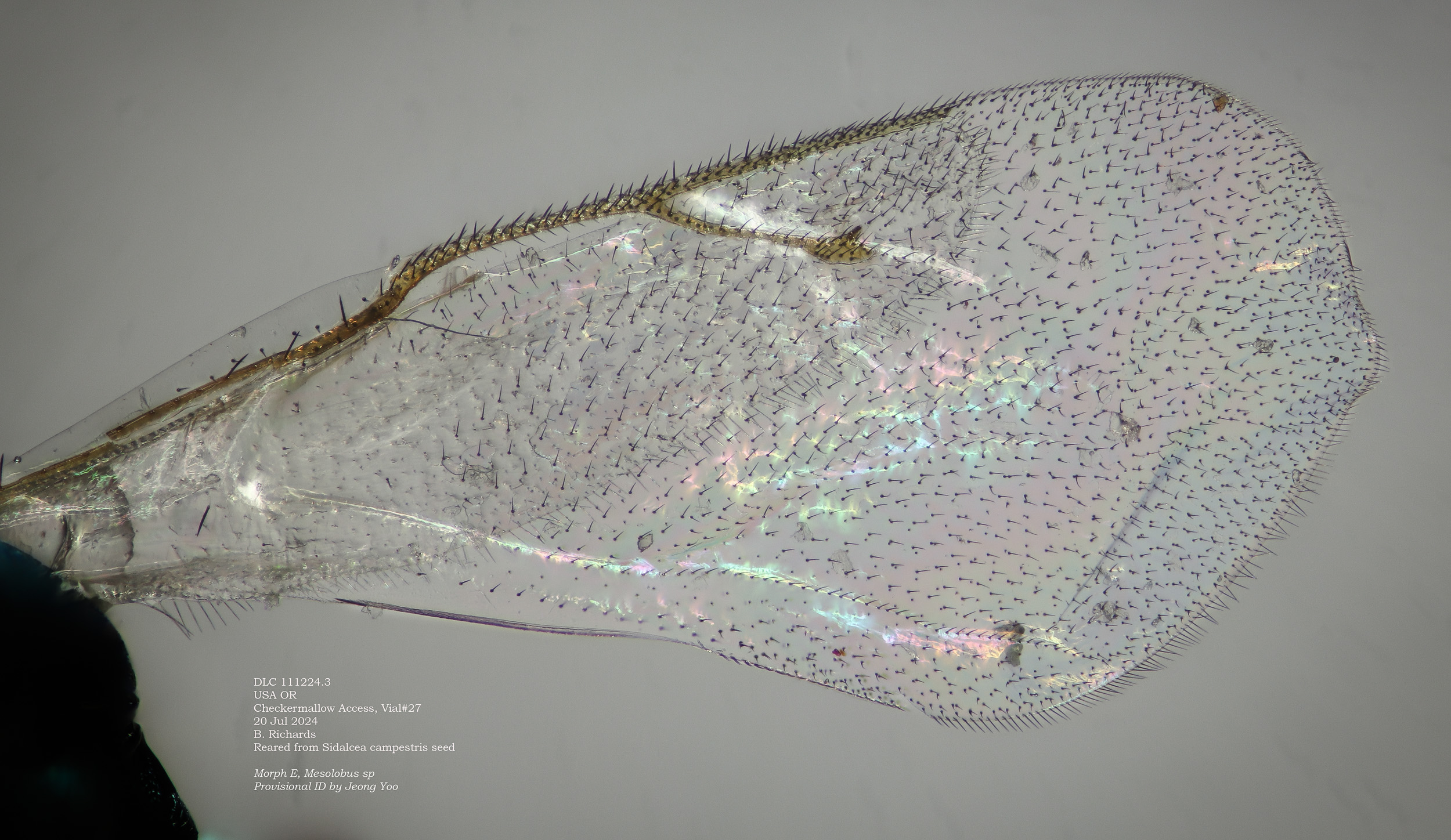
Morph I, with wing detail. Note the longitudinal folds.
|

Morph L, evidently the same as morph I at left.
|

Morph L, ovipositor. Wing with folds.
|
Beginning of a series of specimens from Rufus Isaac's lab at MSU. Note from label info that alternate views may feature different specimens.

♀ Bombus fervidus
|

♀ Bombus fervidus
|

♀ Bombus fervidus
|

♂ Bombus fervidus
|

♂ Bombus fervidus
|

♂ Bombus fervidus
|

♀ Bombus perplexus
|

♀ Bombus perplexus
|

♂ Bombus perplexus
|

♂ Bombus perplexus
|

♀ Bombus vagans
|

♀ Bombus vagans
|

♀ Bombus vagans
|

♂ Bombus vagans
|

♂ Bombus vagans
|

♀ Osmia cornifrons
|

♀ Osmia cornifrons
|

♂ Osmia cornifrons
|

♂ Osmia cornifrons
|

♀ Osmia lignaria
|

♀ Osmia lignaria
|

♂ Osmia lignaria
|

♂ Osmia lignaria
|

♀ Osmia pumila
|

♂ Osmia pumila
|

♀ Osmia cornifrons
|

♂ Osmia pumila
|
CeratinaDetailed treatment of a western Ceratina (females). Click on images for magnified version. C. micheneri at right. C. acantha below. |


|

|

|

|


|

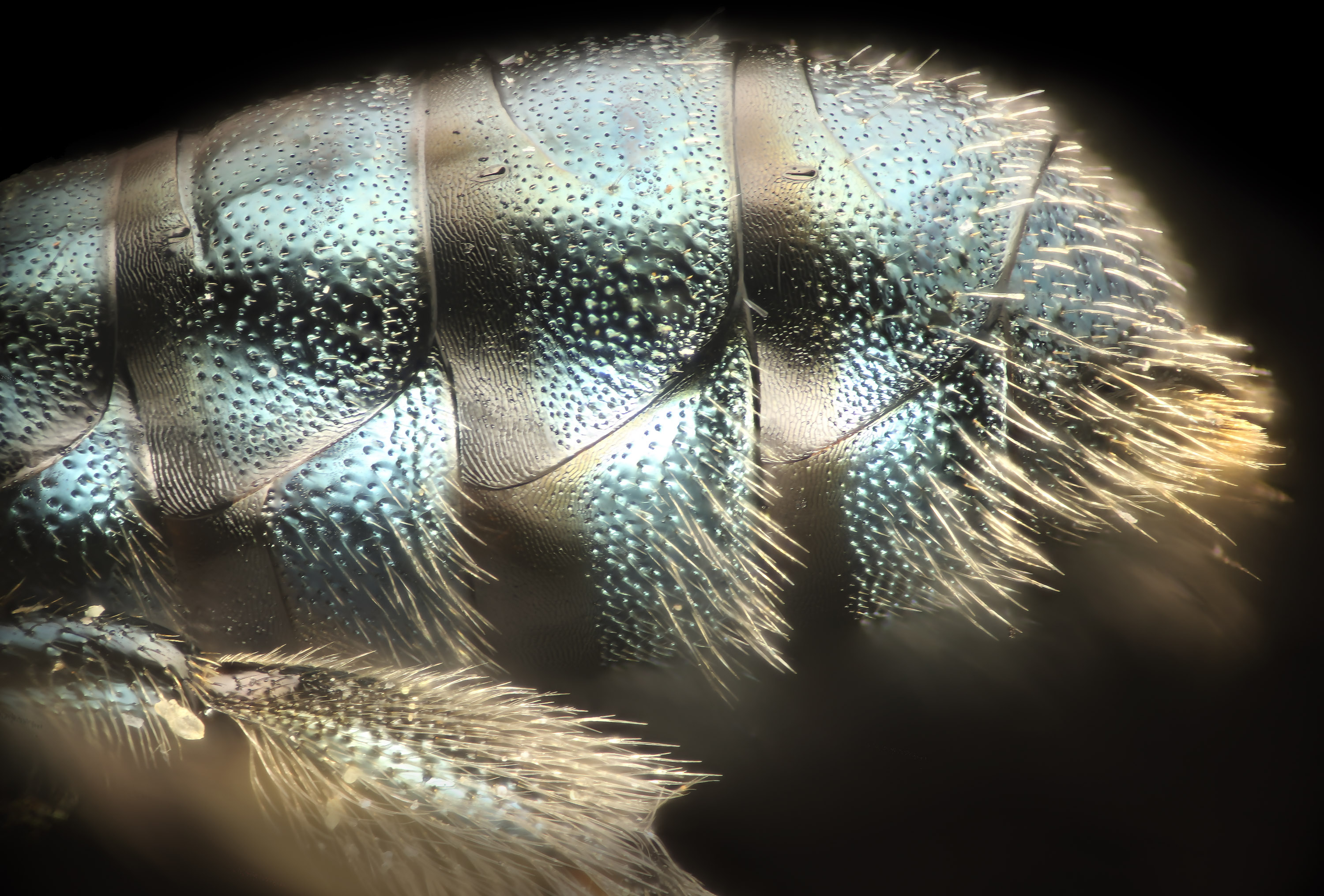
|


|

|

|

|

|
|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
|

|

|

|

|

|

|

|
Bombus of MIB. impatiens is the most abundant MI species (according to iNat). But not so much as I used to suppose. I have to confess that I have historically lumped bimaculatus and griseocollis with impatiens, because they are similar, and because I didn't really pay attention. I've seen a lot of B. grisecollis, now recognize the yellow on T2 and face. |

B. impatiens
|

B. griseocollis (male)
|

B. citrinus, a cuckoo bee.
|

B. ternarius (male), fairly common north in MI.
|
DialictusMy Willamette Valley assumptions about Lasioglossum are inapplicable in SE MI. They are far less abundant. Is this related to the hyperabundance of honeybees? |

L. cressonii

|

L. viridatum

|

Lasio male

|
AndrenaTwo late summer Callandrena species: placata and asteris |

Andrena placata, female
|

Female tergae - shiny and puncate.
|

Andrena placata, male. Face with clypeus yellow.
|

Andrena asteris - this and images to right.
|

|

|
Misc beesAnthidium manicatum 
|

Megachile latimanus

|

Megachile rotundata female above, male below.

|

Triepeolus lunata

|
Odonates, MI vs ORAnother thing that is striking about MI (and the east in general) is the overall abundance of dragonflies and damselflies. These are relatively scarce in the OR Willamette Valley, which gets comparable rain that sustains comparable wetlands. Differences: OR has mild winters, and an extensive dry period. Not clear why either of these would suppress odonates. |

|

|

|

|
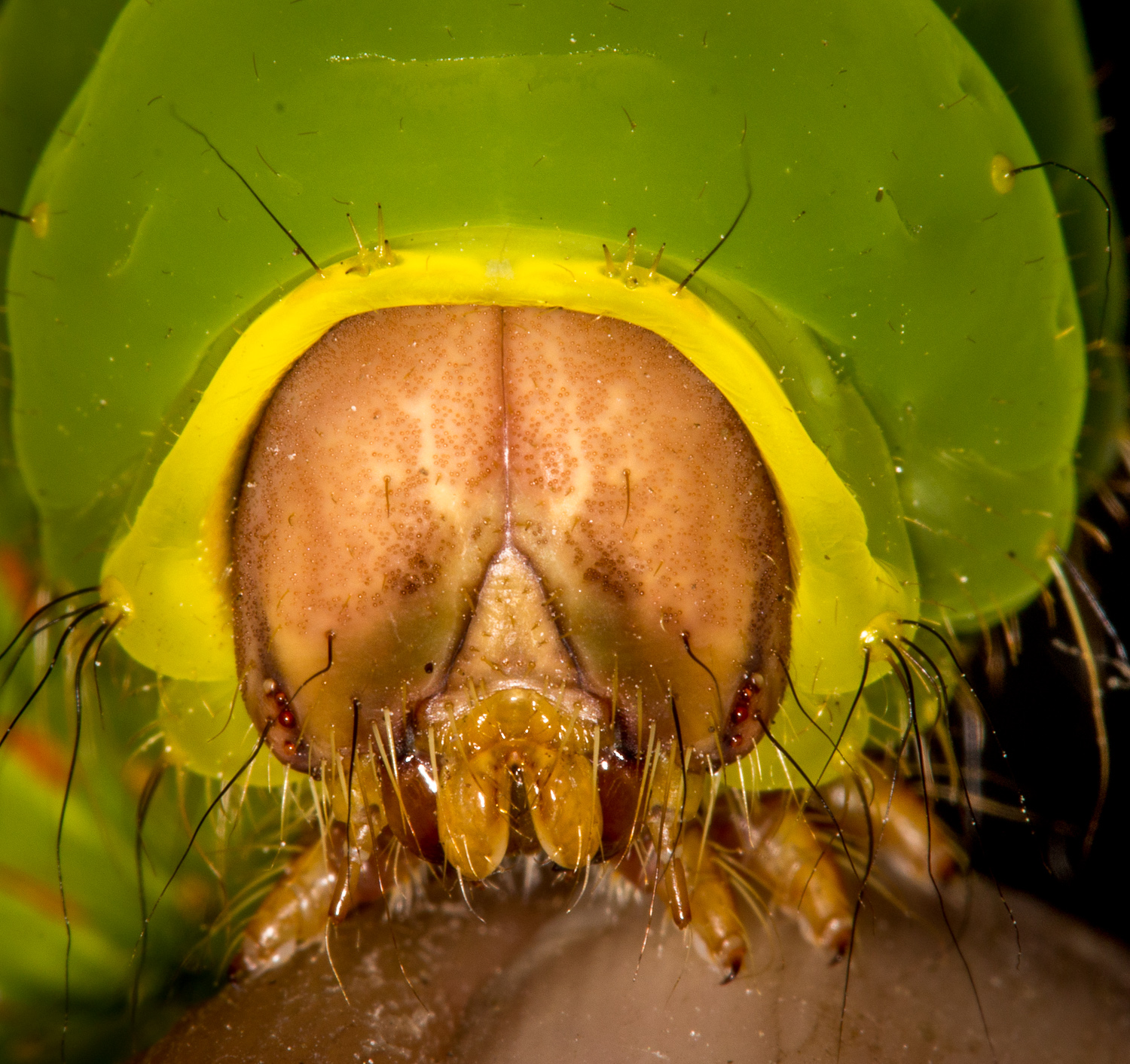
The MountainI helped run a bioblitz for a middle school camp in Highlands NC. |

|

|
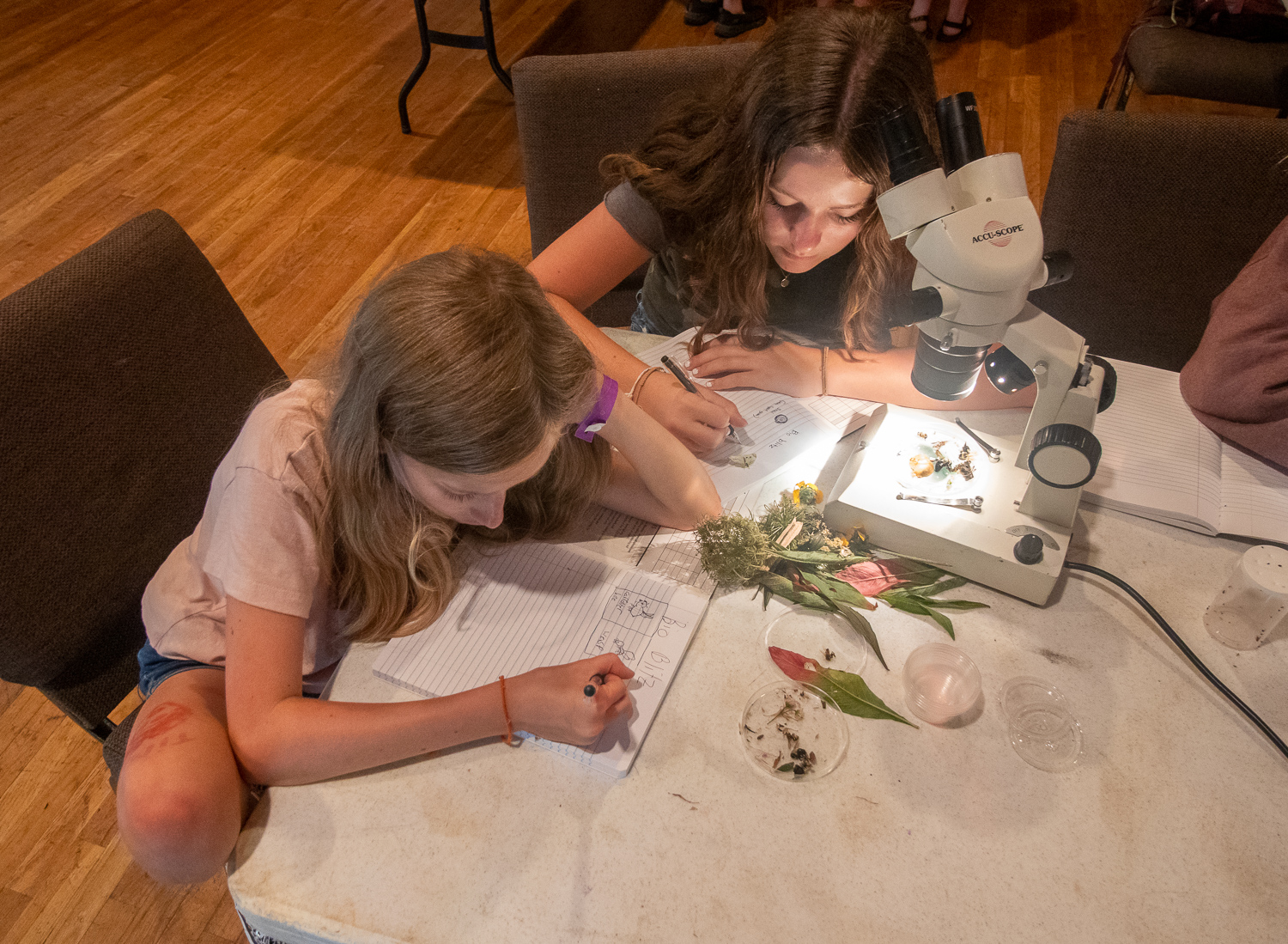
|

|
A more typical M. melanophaeaBelow on this page is the anomalous all-black Megachile melanophaea. This is what melanophaea is supposed to look like. 
|

|
Andrena salicflorisProbably. I include the scutum image at right, which should decide the couplet in the LaBerge key: mesoscutum with anterior third with punctures crowded, separated by mere ridges; vertex above lateral ocellus equals about one ocellar diameter ... salicifloris Cockerell (in part) Mesoscutum with anterior third with punctures discrete, separated by half to almost one puncture width; vertex above lateral ocellus usually distinctly longer than one ocellar diameter .... . miranda Smith (in part). Vertex height is no more than 1 ocd. 
|

|
Recent BombusI am beginning to shoot examples of any PNW Bombus I can get. Because the variation is interesting. And because to my knowledge there is not a reference set of focus-stacked images for these bees. These are from elevation - Mary's Peak, and the Cascades. |

B. flavidus. Two that are clearly the same species. Bottom specimen is yellower - esp mesepisternum, face hairs. Initially I assumed that lack of corbicula was a cuckoo bee thing. But in this case, it is because these are males.

|

Two more males from the same location as photos at left. Top image of B. flavidus is entirly yelow dorsally, and color darker. The bottom image here is also flavidus according to beemachine. But this can't be the same?

|
 Above: Bombus vancouverensis (beemachine ID). Below: B. melanopygus male. Bottom: B. mixtus.


|

Above: the only B. flavifrons I've seen. From Lupine. Below: The mixtus at this location had very dark scutum hairs.

|
B. flavidus male
|

|

|


|
B. melanopygus
|

|

|

|
Andrena nigrihirta
|


|
Lasioglossum sysimbriiL. sysimbrii exhibits white hairs and a distinctive T1 hairband--when it hasn't rubbed off. I have seen a range from "barely there" to unmistakeable. 

|
An entirely black MegachileAny western Megachile I've ever seen has white tergal hair bands. The only black Megachile in the US that I find is Megachile xylocopoides, a southeastern species. UPDATE 22 Jun: best guess, and suggested by Jade Lyf, is M. melanophaea. This would be a very dark variant, for which I find no comparable image. 
|


|
Agapostemon texanus
|
Osmia cyanellaSmall--8.5mm. T2 impunctate to near apical margin, in straight row. Back of head carinate. O. cyanella seems to fit in Rightmyer draft key. Packer image in DL also fits. UPDATE: Molly Rightmyer suggestsn O. cyanella based on the images. |


|


|
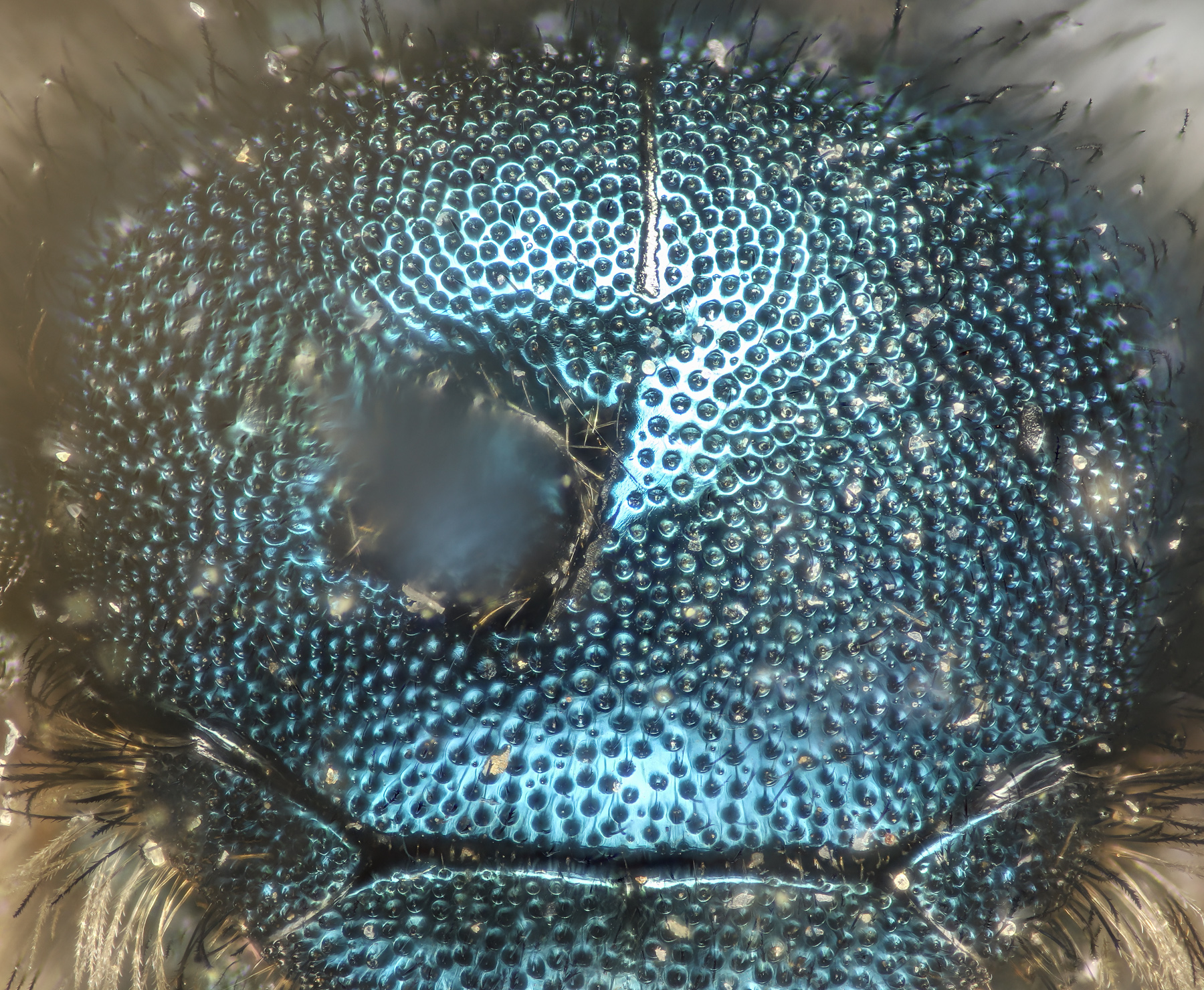

|
May
|

|

|

Osmia cf. albolateralis.
|

Mandilble of specimen left.

|

Osmia trevoris, according to Molly Rightmyer, who notes: (likely a junior synonym of O. inurbana)
|

Notable: apico-medial coloration, clypeal notch.
|


Lupine aphids and predators (syrphid larva and adult) are common.
|

Osmia lignaria
|

Osmia lignaria
|

Hoplitis sp, Herbert Farm.
|

Hoplitis sp, Herbert Farm.
|

Osmia male, Herbert Farm
|

|

|

|

|

|

|

|

|

|

|

|

|

Eucera female. Males are abundant on Sidalcea; not seen on LUPORE
|

Andrena angustitarsata
|

Lasioglossum sysimbrii, Herbert Farm.
|

Meloid larvae on Lasioglossum at left.
|

Osmia atrocyanea (Molly Rightmyer ID from photos) from LUPORE.
|

|

Osmia cf. albolateralis from LUPORE. |

|

|

|

|

|

Osmia male, of the (unknown) species that accounted for 5 of 6 Osmia on river lupine. Notable: large spatulate projection from S3.
|

Above: Osmia male of another (unknown) species on lupine. Below: Protosmia rubifloris.
 |

Face of Protosmia rubifloris, with its decussate mandibles. River lupine.
|

Osmia female on Kincaid's lupine.
|

Above: mandible of bee at left, shot wwith 10X microscope lens. Below: opposite mandible of same bee, shot with 65mm Canon MPE lens.
 |

Face of the Osmia.
|

B. griseocollis on Wyethia at Finley.
|

|

B. californicus on larkspur, at Herbert Farm.
|

B. griseocollis
|

B. melanopygus
|

B. mixtus. Compare to image left - specimen here was washed.
|

B. mixtus again. Same pattern, but hues are different.
|

Pyrrharctia isabella, a tiger moth, from lupine.
|

Chrysidid wasp
|

Bombyliius major, sea blush.
|

Anise swallowtail, lomatium.
|
Apr 24 Herbert Farm. In 2023, I collected a female Ceratina at this site (see March entry) and decided it might be C. tejonensis. Now in 2024, I have collected a few more of these large Ceratina, male and female, at Herbert Farm, and now think C. micheneri is the right call. The T6 character noted below is unclear for me. However the male is NOT tejonensis, based on the metafemur.

Ceratina micheneri female.
|
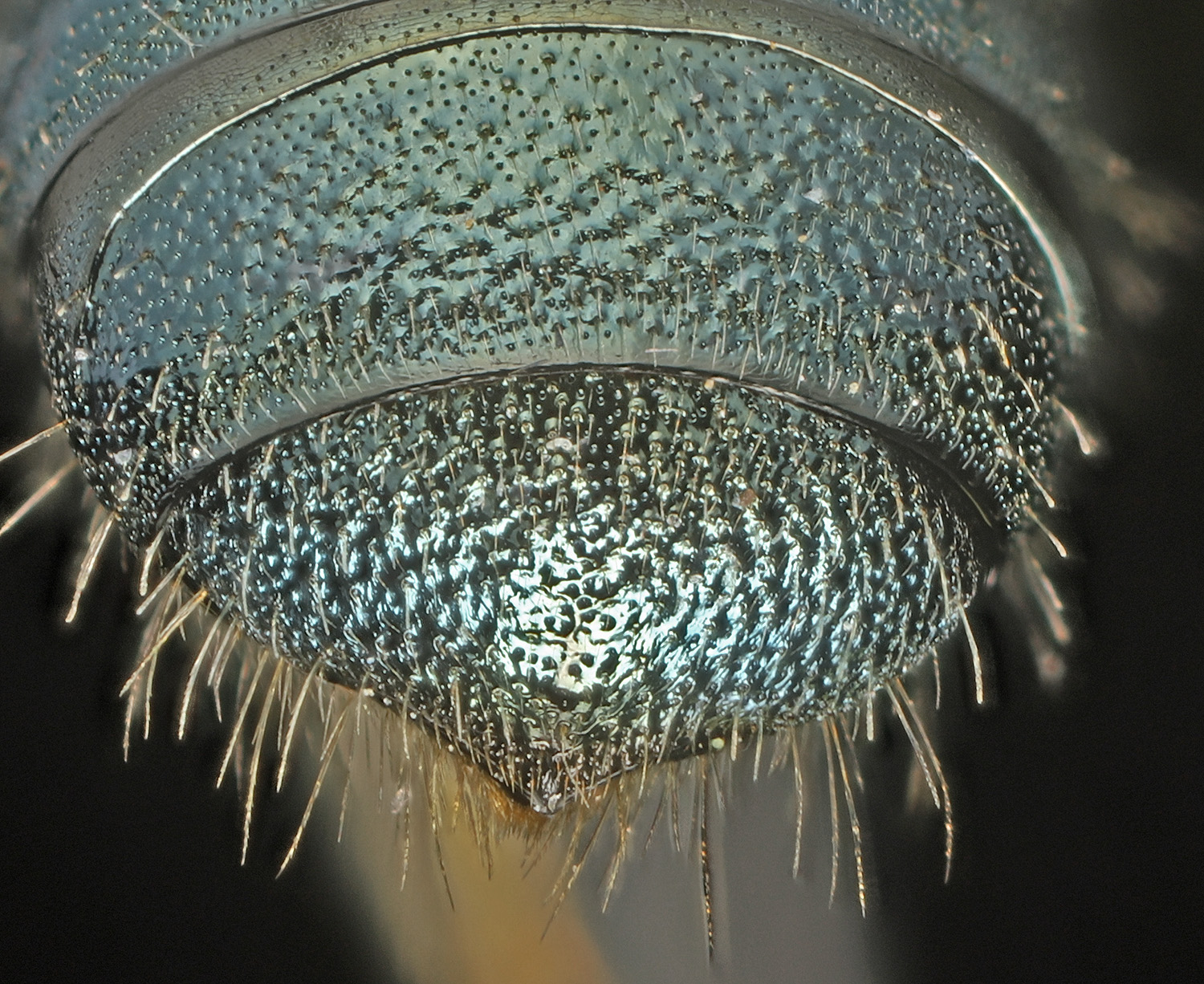
From the Daly key. C. micheneri female: median area of 6th tergum broadly convex, the surface minutely roughened, glistening only near central dish-shaped area which may be non-
metallic or discolored. C. tejonensis: median area of 6th tergum flattened, surface glistening between indistinct punctures, metallic reflection throughout.
|

Ceratina micheneri male. Metafemoral features are a match for micheneri.
|

Andrena cf.salicifloris
|

A. salicifloris: Mesoscutum with anterior third with punctures crowded, separated by mere ridges VS A. miranda: punctures discrete, separated by half to almost one puncture width
|

|
Apr 14 Collected from Camassia quamash.

|

|

As always, diagnosis is not straightforward. Notable: long vertex, ~2 ocd; dense pitting T2. Best fit: A. nivalis.
|
 
|
Apr 10

Melissodes, first of year
|

Agapostemon texanus, first of year.
|

Via both DiscoverLife, and LaBerge Thysandrena keys, I get Andrena w-scripta.
|

I called this A. caerulea. If I am right (possible) and Cody is right (probable), the cf. nigrocaerulea (below, Mar 30) is promoted from cf. nigrocaerulea to caerulea.
|
Apr 1

Male Osmia sp.
|

Male Trachandrena. Dozens flying around, but never touching flowers of small apple tree.
|

|
March 30, Andrena question. In the row below, a specimen that is clearly nigrocaerulea, a match for all character states, and ... obviously nigrocaerulea.

Andrena nigrocaerulea
|

|

|
Compare: Two additional specimens, collected at the same site on the same day also mostly fit nigrocaerulea. That's probably the right ID, but the hairs are off. Episternum, tergae, vertex, clypeus all light, whereas dark is expected for nigrocaerulea. Also, integument lighter, greener.

Andrena caerulea
|

|

|
March 16-18, early season visitors to English daisy, willow, or buttercup; seen at Herbert Farm, or EE Wilson.

Dolichovespula arenaria
|

Dolichovespula arenaria
|

Ichneumoninae
|

Lasioglossum titusi
|


L. titusi, acarinarium and mites.
|

Halictus tripartitus
|

Nomada sp.
|

|

|

Andrena angustitarsata
|

|

|

Andrena male
|

|

|

Andrena male - same as above.
|

Andrena male.
|

Ceratina acantha.
|

Eristalinae (female)
|

Greater Spring Blacklet, Cheilosia grossa.
|

Greater Spring Blacklet, Cheilosia grossa.
|
March 2024. I don't pay much attention to Ceratina in western Oregon. They are hyperabundant. They are almost always one species, C. acantha. Rarely, I see one of the other local species (micheneri, naniula). When I collected the Ceratina below a year ago, I noticed at the time that it was exceptionally big. Sorting specimens recently, I noticed the size again, so looked closely. Ceratina tejonensis. Northernmost record for this species, to my knowledge. One always wonders these days ... climate change?

Original ID: C. tejonensis.
|

Dorsal view of C. tejonensis(?)
|

Mesepisternum pits of C. tejonensis(?)
|
More on putative C. tejonensis Joel Gardner had good questions about this ID. The specimen keys to the two largest Ceratina, tejonensis or micheneri. The two split at:
33(32). Clypeal mark usually present; median area of 6th tergum flattened,surface glistening between indistinct punctures, metallic reflection throughout; cismontane California ... Ceratina tejonensis Cresson
Clypeal mark usually absent; median area of 6th tergum broadly convex, the surface minutely roughened, glistening only near central dish-shaped area which may be non- metallic or discolored; cismontane California, rarely northward ... Ceratina micheneri
The determinative character would be on T6. As so often with keys (not Joel's!) lack of a photo makes interpretation difficult.

C. micheneri from OR Bee Atlas collection: compare to pic above. The stronger bluer shade is a C. micheneri trait. Clypeal mark absent. The pit distribution appears different.
|

I don't have a C. micheneri specimen to examine, so I can't make a comparison. This pic is T6 of the tejonensis (?). Does it fit?
|

|

|

|

|
David Cappaert, Update 27 Apr 2024